Quality of Manufacturing
Our commitment to quality – manufacturing to the highest standard
As with our ingredients and formulations quality drives the entire product creation journey, and is guaranteed by our dedicated Quality Assurance (QA) team.
 The Manufacturing Journey
The Manufacturing Journey
The manufacturing journey begins and ends with our QA team. Ingredients are tested as soon as they enter the door and will continue to be monitored through every step of the process.
 Ingredient weighing
Ingredient weighing
Our Quality Assurance team initiates production by preloading our automated weighing system with the appropriate formula for the desired product. The weighing of the ingredients is then computer controlled, virtually eliminating any possible human error. Even so, following weighing, the QA team examines the log from the scale to verify that all ingredients were weighed correctly.


“You might think it’s crazy for us to double check something that can’t be wrong? Maybe. But it is imperative that this critical step be absolutely correct.”
 Blending
Blending
Weighed ingredients are then transferred into our custom made stainless-steel totes. Quality Assurance verifies that the number of totes is consistent with the stipulated batch size. The totes are then moved to one of our various stainless-steel mirror-finished blenders. Over the years, with the growth of the company, we have added to our collection of blenders, with our largest now at 150 cubic feet. To put this in perspective, this large blender will hold just under 4,250 litres of liquid and is one of only a handful this size in the United States.
Once blended, QA samples the mixture and fingerprints it with our FTIR and compares it to the standard for that formula. If it passes, the mixture is again put into totes, which QA verifies are the proper number and weight. The totes are now ready to be moved to production stations.

Production Stations / Product Forms
The totes are either transferred directly to the powder-filling line for bottling, or if a tablet or capsule is to be made, they are transferred to the appropriate production station.
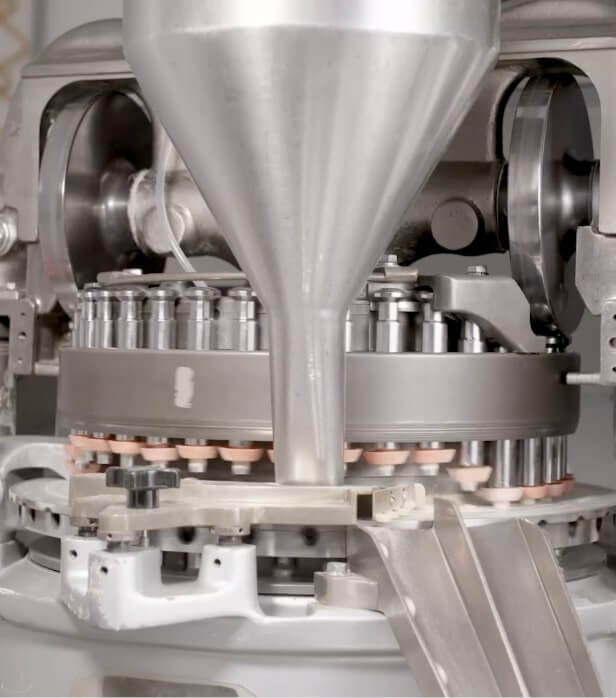
 Tableting
Tableting
While we have multiple tablet presses in the facility, it is our two German high-speed, computer-controlled tablet presses capable of producing over 200,000 tablets per hour that see the most use. These presses are 2-5 times faster than the others and allow us to press over 3,300 bottles of Proanthenols® per hour.
Every 60 minutes during tableting, someone from QA will collect a sample to confirm that the press is still producing according to specification, notifying the operator if any adjustments are necessary. Samples taken during tablet production are assessed for size, durability, and dissolvability. If samples do not pass, processes are stopped, products not up to our specifications are discarded, any errors identified are corrected, and only then will production be allowed to proceed.
 Encapsulating
Encapsulating
While not quite as fast as our tableting machines, our current encapsulation machine can produce up to 70,000 capsules per hour allowing us to produce over 1,100 bottles of Aloe Vera Caps per hour. As in tableting, samples of capsules are taken every 60 minutes.


 Bottling
Bottling
We bottle our products using custom designed, automated, high-speed lines regardless of whether we are bottling our powders, tablets, capsules or soft gels. During bottling, QA pulls samples every 60 minutes.
While the exact order of the steps varies based on product form, each bottle of product is:
 Further Testing
Further Testing
Our production may be finished but quality checks continue.


Cleanliness of Machinery
Once a batch of product is complete, the machinery is thoroughly cleaned, and then the Quality Assurance team will come through one more time to confirm that there is no trace of product OR any cleaning agents using our Total Organic Carbon (TOC) testing machine. Again, giving us the ability to be confident in our commitment to producing the cleanest products possible.
Product Testing & “Best Before” date
Samples from each and every batch of product are retained in order to confirm potency and purity for the entire lifespan through the “Best Before” date. We do not ‘skip-test’.
Quality program checks by the FDA
Our policies and procedures are audited and regulated by the US Food and Drug Administration (FDA) current Good Manufacturing Practices (cGMP) program; which means we are subject to regular unannounced audits to ensure ongoing compliance.
Our commitment to you; quality comes first
Quality is not a box-ticking exercise for us. It’s an obsession and one of which we are proud. We will never compromise our ethics or standards. That’s why you can rest assured you’re only ever getting the best.